Abstract
Background: Despite numerous treatment strategies, failure rates remain 25-30% for pediatric ALCL. Brentuximab vedotin is an antibody-drug conjugate containing an anti-CD30 monoclonal antibody linked to a tubulin inhibitor (monomethylauristatin E) which has significant activity as single agent in relapsed ALCL. The primary aim of ANHL12P1 (NCT01979536) is to determine the toxicity and efficacy of the addition of two novel agents (brentuximab vedotin or crizotinib) to standard chemotherapy (best arm of ALCL99) in children with newly diagnosed, non-localized, ALK+/CD30+ ALCL.
Methods: Patients on Arm BV received standard chemotherapy plus brentuximab vedotin. All patients initially received a 5 day prophase followed by 6 cycles of chemotherapy (dexamethasone, ifosfamide, cyclophosphamide, doxorubicin, etoposide, cytarabine, methotrexate) at 21 day intervals. Brentuximab vedotin was given on day 1 of each of the 6 cycles excluding prophase.
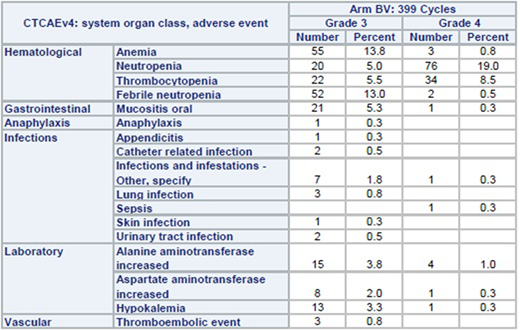
Results: From November 2013 to January 2017, 68 children with ALCL were enrolled on Arm BV. One patient was taken off protocol at the treating physician's discretion during prophase before receiving brentuximab vedotin leaving 67 eligible patients for toxicity evaluation. 66 of 67 patients completed all 6 cycles of chemotherapy resulting in 399 cycles evaluable. There were no toxic deaths, no cases of progressive multifocal leukoencephalopathy syndrome, and no cases of grade 3 or 4 neuropathy. Grade 3 or 4 toxicities which occurred in more than 5% of cycles included hematological toxicities, mucositis, and febrile neutropenia (see table). The mean/median interval between each cycle was: 22.7/22 days (cycle 1 to 2), 22.7/21 (2 to 3), 22.8/21 (3 to 4), 23/21 (4 to 5), and 22.8/21 (5 to 6). Interval between cycles was >28 days in only 3.6% of all cycles.
Conclusions: Arm BV of ANHL12P1 demonstrated that the addition of brentunixmab vedotin to standard chemotherapy does not cause significantly added toxicity. Brentuximab vedotin did not change the desired interval between cycles with the interval remaining similar from first to last cycle. It remains to be seen if the addition of brenutuximab vedotin improves overall or event-free survival compared to historical controls.
Bollard:Cellectis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Torque: Honoraria, Membership on an entity's Board of Directors or advisory committees; Neximmune: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal